
Photo: PictureDesignSwiss/Depositphotos
Alzheimer’s disease is a devasting neurological disorder that affects memory, thinking, and behavior for which there is no cure. However, there is some encouraging news for those with Alzheimer’s and their families. Pharmaceutical companies Eisai and Biogen have worked together to create lecanemab, a drug that has been designed to slow cognitive decline in people with dementia and Alzheimer’s. The results from their human trial are positive; while experts are cautious, the drug could change the future of treatment of those with cognitive decline.
The human trial comprised 1,795 participants with “mild cognitive impairment” from Japan, China, the U.S., and Europe. For 18 months, half of the participants were given bi-weekly infusions of the drug while the other half received a placebo. Using the Clinical Dementia Rating-Sum of Boxes (CDR-SB), “a numeric scale designed to quantify the severity of dementia,” participants’ cognitive decline was monitored. Results show that the drug “slowed progress of the disease by 27% compared with a placebo.”
Lecanemab was created based on the theory that a protein called amyloid beta is the cause of neurodegeneration associated with the disease. The drug, an intravenous antibody, binds to and removes these toxic proteins. Biogen’s previous Alzheimer’s drug, Aduhelm, worked to do the same—although it was not nearly as successful. Unlike Aduhelm, which only attacked clusters of amyloid, lecanemab “targets forms of amyloid that have not yet clumped together.”
Researchers say the study proves the theory that the removal of amyloid proteins slows down the harmful disease and that these results are “highly statistically significant.” But other experts remain cautious to accept these conclusions fully. Ronald Petersen, director of the Mayo Clinic Alzheimer’s Disease Research Center in Minnesota stated, “It’s not a huge effect, but it’s a positive effect.”
Rob Howard, a professor of Old Age Psychiatry at University College London, agrees that the results are statistically historic, specifically because they are the first clinical trial evidence of a drug slowing the progression of dementia symptoms. He identifies, though, that the results do not prove the efficacy of the drug on a wide-scale, real-world basis. Howard cites the CDR-SB results, stating there was only, “A 0.45 point advantage on an 18-point scale, where the accepted minimum worthwhile difference ranges from 0.5 to 1.0 points…” The professor continues on to say these results, “will mean that there are going to be some very difficult conversations and decisions in the next weeks and months.”
Other experts have suggested that the anti-amyloid treatment is only addressing part of the problem. Dr. Kristian Steen Frederiksen, director of a clinical trial unit at the University of Copenhagen, states that Alzheimer’s “is an extremely complex disease and amyloid-related pathology is unlikely to be the only player. Therefore targeting a single target is not likely to produce large effect sizes.”
While there are those that doubt the efficacy of lecanemab, especially on a large scale, this could mean major relief and hope for those with dementia. Tara Spire-Jones, University of Edinburgh, emphasizes, “While this is not a ‘cure’ in that it doesn’t bring people back to normal, slowing cognitive decline and preserving the ability to perform normal daily activities would still be a huge win because people could live well for longer with Alzheimer’s disease.”
Eisai is seeking FDA approval “under the accelerated approval pathway” and a decision is set to be made in January 2023.
Eisai and Biogen have released positive results from their human trial of their drug lecanemab, an intravenous antibody designed to slow the cognitive decline of those suffering from dementia and Alzheimer’s.

Photo: postmodernstudio/Depositphotos
Lecanemab binds to and removes proteins called amyloid beta, which is thought to cause neurodegeneration associated with the disease.
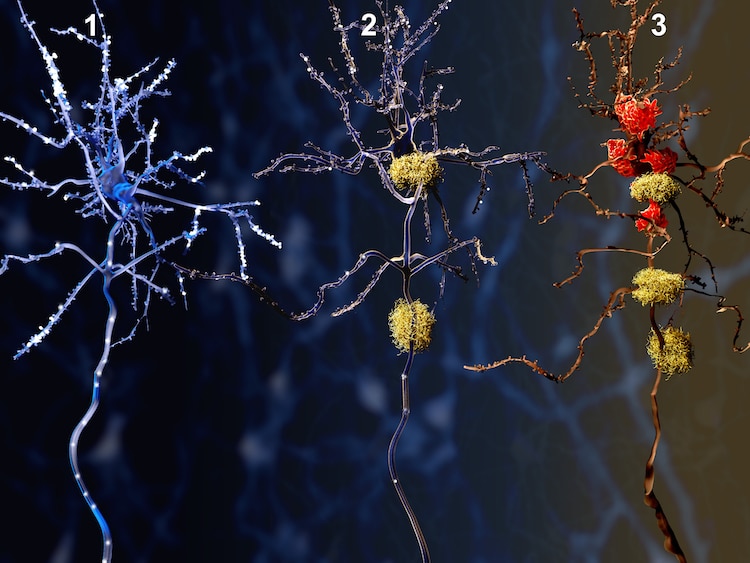
Illustration of three stages of Alzheimer’s, a healthy neuron slowly dying from amyloid proteins. Photo: animaxx3d/Depositphotos
The trial comprised 1,795 participants with “mild cognitive impairment” from Japan, China, the U.S., and Europe. For 18 months, half of the participants were given bi-weekly infusions of the drug, while the other half received a placebo.
Photo: IgorVetushko/Depositphotos
Results of the trial show that the drug slowed the disease’s progression by 27% compared to the placebo.

Photo: AndrewLozovyi/Depositphotos
While some experts say these results do not prove the efficacy of the drug on a wide-scale, real-world basis and do not prove the drug is a cure, many state these results do mean there is hope for the preservation and prolongation of life of those with dementia and Alzheimer’s.

Photo: Kubko/Depositphotos
Eisai is seeking accelerated FDA approval and a decision is set to be made in January 2023.

Photo: grandbrothers/Depositphotos
h/t: [NewsAtlas]
Related Articles:
New Algorithm Accurately Spots Early Signs of Brain Changes Before Alzheimer’s Disease
Former Ballerina With Alzheimer’s Hears Music and Remembers Her Routine To ‘Swan Lake’
Horse Keeps Running Away to Visit Dementia Patients Who Feed Him Carrots
